- Free Test Series, Mock tests and Practice Tests
- Time proven exam strategies
- Exam analysis and simulated tests
- Hand-on real time test experience

Recently Added Articles View More >>
Welcome to our comprehensive guide on Science General Knowledge (GK) for SSC exams! If you're gearing up for the SSC CGL, CHSL, or any other competitive exams, you know how crucial a strong grasp of science concepts can be.
Welcome to the General Science Quiz with Answers blog, your ultimate destination for enhancing your scientific knowledge through fun and engaging quizzes! Whether you're a student, teacher, or lifelong learner
Welcome to our site for the Science GK Quiz with Answers Blog! This website is the best place to learn about the fascinating field of science by taking interesting quizzes.
Welcome to our General Science Quiz and Answers Blog, where curiosity meets knowledge! Here, we delve into the fascinating world of science, covering a broad spectrum of topics ranging from biology and chemistry to physics and beyond.
Welcome to our comprehensive guide designed to help you conquer basic science quizzes for competitive exams. Whether you're preparing for entrance tests, job interviews, or academic competitions, having a strong foundation in basic science is essential.
Boost Your Scientific Skills: Dive into this Science GK Quiz for Competitive Exams! Are you ready to test your knowledge in the world of Science GK Quiz? Delve into this captivating quiz designed to challenge your understanding of various scientific disciplines.
Welcome to our Basic Science GK Quiz blog, where curiosity meets knowledge! Dive into the fascinating world of science with our collection of engaging quizzes designed to test your understanding of fundamental scientific concepts.
Welcome to our General Science Question and Answer blog, where curiosity meets knowledge! Here, we explore the fascinating world of science, unravelling its mysteries one question at a time.
Most Popular Articles
Most Popular Articles
Recently Added Questions
- 1If it has more electrons than protonsfalse
- 2If it has more electrons than neutronstrue
- 3If it has more protons than electronsfalse
- 4If it has more protons than neutronsfalse
- Show Answer
- Workspace
- SingleChoice
Answer : 2 If it has more electrons than neutrons
Explanation :
If an atom has more electrons than protons, it is a negative ion or anion. If it has more protons than electrons, it is a positive ion.
Which of the following elements is NOT a component of baking soda?
752 1 64bfc857a2d4dcaf044da339- 1Hydrogenfalse
- 2Calciumfalse
- 3Sodiumfalse
- 4Oxygentrue
- Show Answer
- Workspace
- SingleChoice
Answer : 4 Oxygen
Explanation :
1. Which of the following calcium elements is not a component of baking soda?
2. The chemical formula of baking soda is NaHCO3, which is composed of four elements sodium, hydrogen, carbon and oxygen.
3. Baking soda is an alkaline substance used as a leavening agent in baking.
Which law was studied in the year 1787, in which it was said that the volume of a gas increases with its absolute temperature and if its absolute temperature decreases, then its volume will decrease?
711 0 64b5204188d5e4f52dd2277b- 1Boyle’s lawfalse
- 2Dalton’s lawfalse
- 3Avogadro’s lawfalse
- 4Charles's lawtrue
- Show Answer
- Workspace
- SingleChoice
Answer : 4 Charles's law
Explanation :
1. Charles's law (also known as volume law) is an experimental gas law.
2. The volume of a gas increases with its absolute temperature and as its absolute temperature decreases its volume also decreases.
3. Charles's law was studied in the year 1787.
- 1Alcoholtrue
- 2Ethanefalse
- 3Ketonefalse
- 4Aldehydefalse
- Show Answer
- Workspace
- SingleChoice
Answer : 1 Alcohol
Explanation :
1. That class of compounds, which contain a functional group –OH, is called alcohol.
2. Alcohols contain a hydrogen atom and a hydroxyl group (-OH) along with a carbon atom.
3. The hydroxyl group is a polar group, which makes the alcohol soluble in water.
Hydrogen resembles the properties of which two groups of the periodic table?
644 0 64a2b0fac7d7c7e0674a2640- 1Group 2 and Group 17false
- 2Group 1 and Group 3false
- 3Group 1 and Group 17true
- 4Group 2 and Group 4false
- Show Answer
- Workspace
- SingleChoice
Answer : 3 Group 1 and Group 17
Explanation :
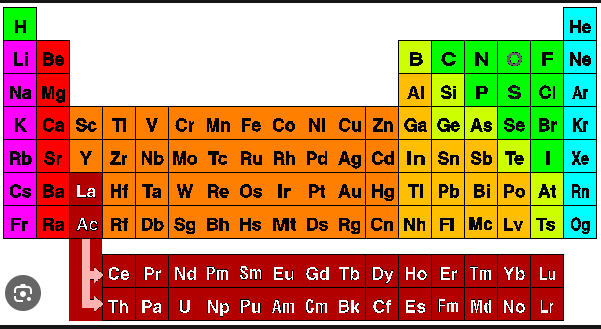
2. Following is some additional information about the properties of hydrogen.
- Hydrogen is a highly flammable gas.
-Hydrogen is a major component of water.
- Hydrogen can be used as fuel.
Hydrogen is used in the chemical industry to make many types of products.